Abstract
Introduction: T-MN inclusive of therapy related myelodysplastic syndrome (T-MDS) and acute myeloid leukemia (T-AML) are aggressive neoplasms occurring after exposure to chemo (CT) and/or radiotherapy (RT) and are characterized by high frequency of complex cytogenetics and poor prognosis. TP53 and other genes involved in DNA repair such as Fanconi Anaemia(FANC) pathway are critical in maintaining genomic stability. TP53 mutations are associated with complex karyotype and poor prognosis in myeloid malignancies. Further, variants in the FANC pathway are a risk factor for malignancies, but remain less well studied in T-MN. Churpek et al . (Cancer 2016) reported germline mutations in BRCA1, BRCA2, TP53, CHEK2 and PALB2 in 8/43 (19%) treated breast cancer patients who developed T-MN. However, their interaction with TP53 mutations and impact on survival is unknown. Here we investigate the genomic architecture of T-MN patients from the South Australian Myelodysplastic Syndrome (SA-MDS) registry.
Methods: Demographic, clinical and laboratory data including cytogenetic profile of 147 T-MN patients were analysed. Targeted Massively Parallel Sequencing of a custom panel of 63 genes (43 myeloid neoplasms associated genes & 20 FANC; all coding regions) was performed on diagnosis bone marrow samples from 101 T-MN samples. Rare FANC variants were selected based ExAC MAF <0.2% and variants only with VAL ≥20% were selected for further analysis. FANC variants were called deleterious if predicted to be deleterious by atleast two in-silico prediction model (e.g. CADD, SIFT, POLYPHEN and Mutation taster) and/or reported in COSMIC, gene specific databases or in literature.
Results: Median age of T-MN was 71 (20-91) years and the most frequent primary neoplasms were lymphoproliferative neoplasms (n=56, 38%) and prostate cancer (n=22, 15%). Sixty-three (43%), 34 (23%) and 48(33%) patients received CT, RT and CT plus RT respectively. The T-MN group consisted of 104 T-MDS (71%) and 43 T-AML (29%). Poor risk cytogenetics were more frequent in T-MN cases following CT than after RT alone.
Somatic mutations in myeloid genes were detected in 95/101 (94%) of T-MN cases. Notably, all normal karyotype T-MN (29/29, 100%) were mutated. Mutations were most frequently seen in transcriptional factors (35%), TP53 (32%) and splicing factors (26%). Significantly higher proportion of TP53mut T-MN patients had complex karyotype (22/32, 84%) compared to TP53wt (5/69; 7%, p<0.0001).
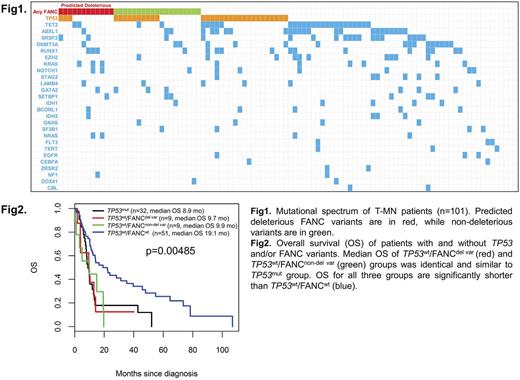
Examination of 20 FANC pathway genes identified 38 rare variants in 31(31%) T-MN at a variant allele frequency (VAF) of ≥20% and most of the variants were missense (35/38; 92%). These were mostly seen in BRCA1, FANCA, FANCM, BRCA2, FANCL and SLX4 genes. Of patients with FANC variants, 42% (13/31) had concomitant TP53 mutations. The median VAF was 49% (range 20-100%) with no significant difference between the deleterious (n=13; 34%) and non-deleterious variants (n=25). Out of 13 deleterious variants (including 2 BRCA1 variants previously reported pathogenic on BIC database), ten variants were seen in nine TP53wt patients. We compared the disease characteristics, prior chemotherapy and survival outcome in patients with TP53mut (n=32), TP53wt harbouring predicted deleterious FANC variants (TP53wt /FANCdel var; n=9), TP53wt harbouring predicted non-deleterious FANC variants (TP53wt /FANCnon-del var; n=9) and TP53wt /FANCwt (n=51). There was no difference in bone marrow blast %, type of prior malignancy, prior therapy (CT vs RT vs combination), proportion of T-AML and treatment for T-MN between the groups. Interestingly, median number of mutations were significantly lower in TP53mut group (1; range 0-11) compared to TP53wt /FANCdel var, TP53wt /FANCnon-del var (both groups - 3; range 0-6) and TP53wt /FANCwt (3; range 0-9) (p=0.0005). Importantly, median OS of TP53wt /FANCdel var and TP53wt /FANCnon-del var groups was identical and significantly shorter than TP53wt /FANCwt (9.7 vs. 9.9 vs. 19.1 months; p=0.00485), and similar to TP53mut group (8.9 months).
Conclusions: In our cohort, somatic mutations were seen in 90% of T-MN patients, comparable to primary MDS, contrary to previously published literature. Somatic TP53 mutations and FANC variants are seen in 32% and 31% of cases respectively and 13% harboring both. This study demonstrating that rare FANC variants are associated with significantly poor survival comparable to TP53 mutated T-MN patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal